Test methods beyond the accredited range
Beyond the accredited testing range, the test laboratory offers further methods validated within by the quality management system, which have proven to be successful especially in comparative investigations of newly developed products or products available on the market.
These tests can be adapted particularly flexible to the demands of clients, without leaving the quality standards of the test laboratory. A list of the methods currently available is given in the following:
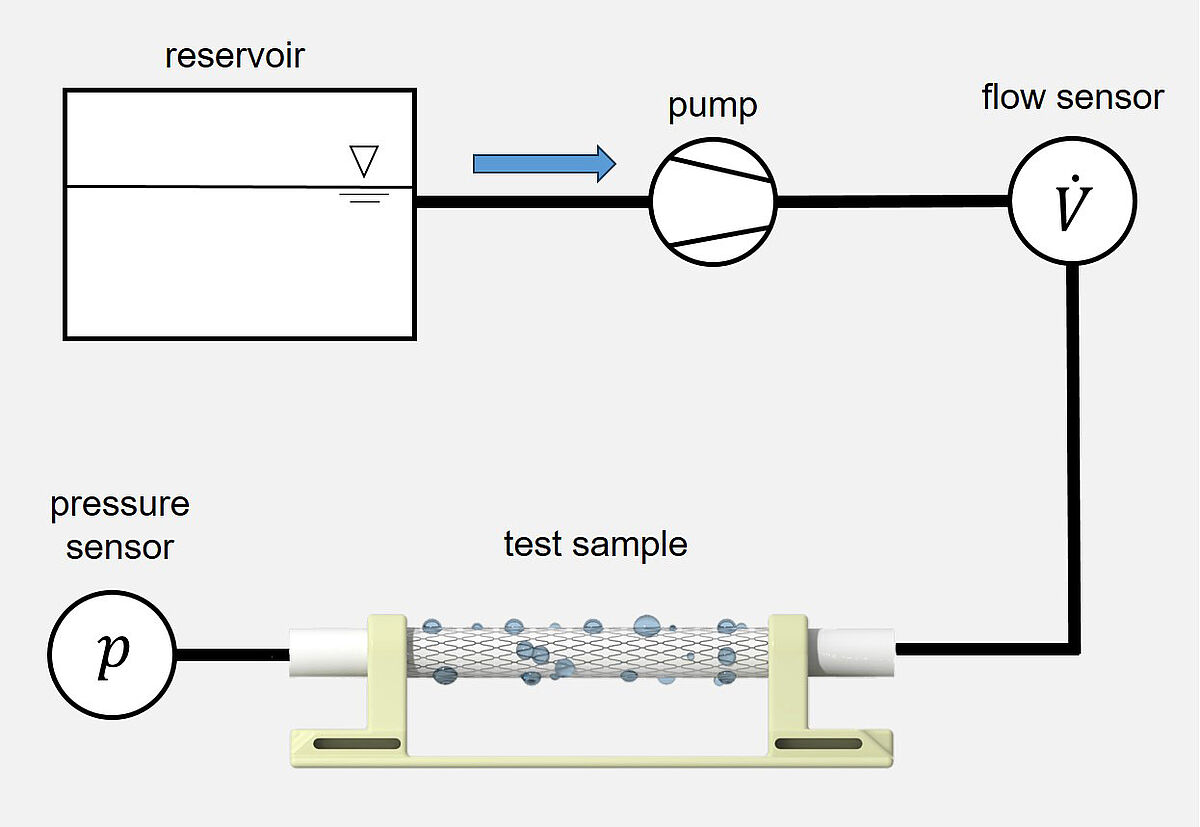
Measurement of the integral water permeability, water entry pressure, and maximum tolerable internal pressure of a vascular prosthesis
Normative reference of the test procedure:
DIN EN ISO 7198:2017, sect. 8.7.2.1.2, 8.7.2.1.5, A.5.1.3 A.5.1.4
The test procedure covers determining the integral water permeability (at an internal pressure of 120 mmHg), water entry pressure, and maximum tolerable internal pressure of a (covered) vascular prosthesis. During application of a defined internal pressure the amount of water exiting per time unit through the outer wall of the (covered) vascular prosthesis is measured. This pressure is stepwise increased by 30 mmHg until the maximum tolerable internal pressure.
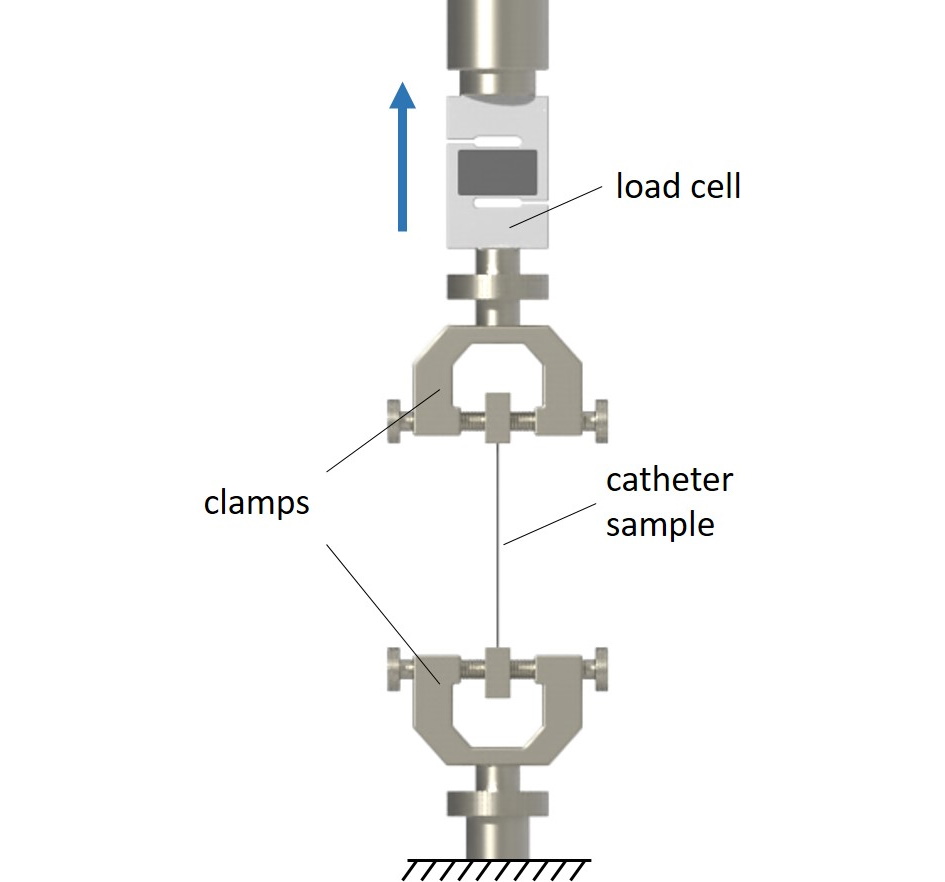
Peak tensile force of catheters
Normative reference of the test procedure:
ISO 10555-1:2023, sect. 4.9 and annex B
The peak tensile force of catheters is determined according to ISO 10555-1:2023, section 4.9 and Annex B. Tests may be conducted either on the complete catheter or on various (or relevant) catheter segments. The specimens are investigated in a uniaxial tensile test. The determined peak tensile forces are evaluated according to the minimum values defined in ISO 10555-1:2023.
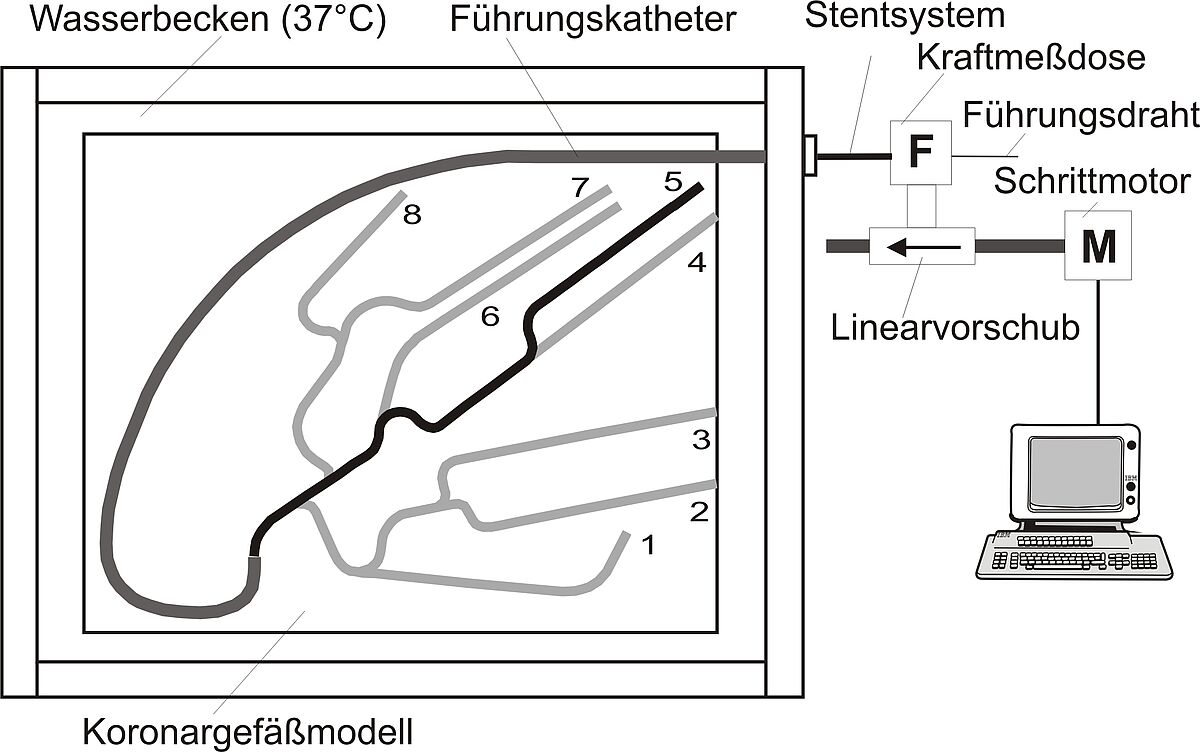
Trackability of balloon catheters and stent systems
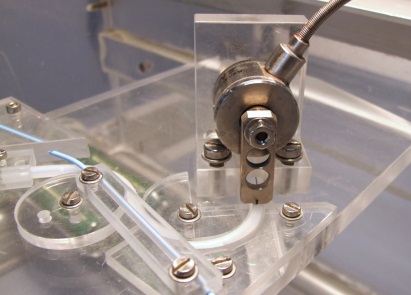
A test setup consisting of a linear drive with mounted load cell and a specific vessel model within a heated water bath is available to assess the trackability of balloon catheters and stent systems The force recorded during the advancement of the test systems gives information on the ease of delivery through narrow tortuous vessels. The equipment is well capable to compare different commercially available products. It can be used in addition to the standardized simulated use testing.
Pushability of balloon catheters and stent systems
To assess the pushability of balloon catheters and stent systems the same test setup is used as for trackability testing. It is completed by another load cell at the distal end of the vessel model to measure the reactive force of a total obstruction. The result is the relation of the distally measured force and the proximal applied push force (pushability in %) The equipment is well capable for comparison of different products.
X-ray contrast of medical products
Normative reference of the test procedure:
DIN EN ISO 25539-2:2021, sect. 8.5.1.13 and D.5.2.11.2,
ASTM F640-20,
DIN 13273-7:2020
The test scope is to determine the X-ray attenuation of the test samples using a setup according to DIN 13273-7:2020 and enables a comparative assessment of X-ray visibility.
The test samples are placed on a scattering phantom together with a reference sample (Aluminum steps). As measure of X-ray attenuation the difference in gray scale values of the test samples and the film as well as the corresponding aluminum thickness dAl are provided.